Research
Cell Cycle Regulation
The Cell Cycle Regulation laboratory is interested in general principles of biology concerning the assembly and maintenance of complex subcellular structures, as well as their variations, which can occur during development, disease and evolution. Researchers use complex cytoskeletal assemblies, such as centrioles and cilia, as study subjects. Centrioles are microtubule-based cylinders that form centrosomes and cilia, structures involved in many functions, from cell division to motility. Centrosome defects are seen in many cancers, while abnormalities in cilia lead to many diseases including polycystic kidneys and infertility.
-
Centrosomes and Cilia
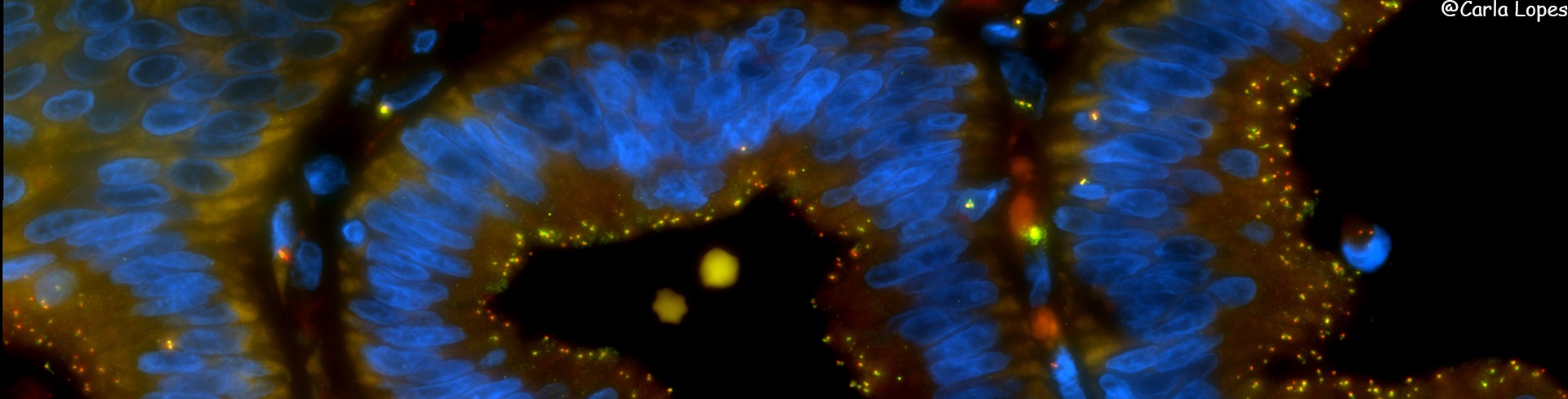
View the embedded image gallery online at:
https://sites.igc.gulbenkian.pt/ccr/index.php/research#sigProId5823aafd0aCentrosomes and cilia are two cellular structures that are critical for many cell functions. Centrosomes are the major microtubule organising center in animal cycling cells, participating in cell division and polarity. Cilia are cellular protrusions involved in cellular signalling and movement. In common, both are made of centrioles, a small microtubule-based cylinder.
During centrosome assembly, the two centrioles of the centrosome associate with the pericentriolar matrix (PCM), which helps to nucleate and anchor microtubules. During cilia formation, one centriole, called basal-body, tethers to the membrane and grows the axoneme, a MT-based structure that makes the skeleton of the cilia.
For a recent review see: Nabais et al, 2018.
- 1
-
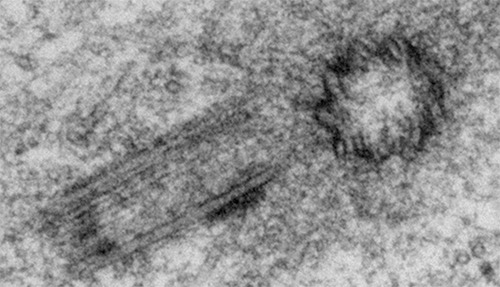
Biogenesis and function
It is fascinating how the formation of new centrioles is highly regulated in animal cycling cells: once every cell cycle, one new “daughter” centriole grows close to the already existing one, the “mother”, ensuring maintenance of centriole number. In certain cases, centrioles can also form with no “mother”, i.e. de novo. Centriole biogenesis raises many interesting questions also relevant for other structures in the cell: how is time, place and number is regulated.
Our recent work and the one of others highlighted several properties of the system, including positive and negative feedbacks, cell cycle and spatial cues (e.g. Ito et al, eLife, 2019, Cunha-Ferreira et al, Curr Biol, 2013, Lopes et al, Dev Cell 2015, Zitouni et al, Curr Biol, 2016, Gouveia et al, JCS, 2018). We are exploring critical hypotheses through a combination of biochemistry, genetics, quantitative live cell microscopy and computational modelling.
-
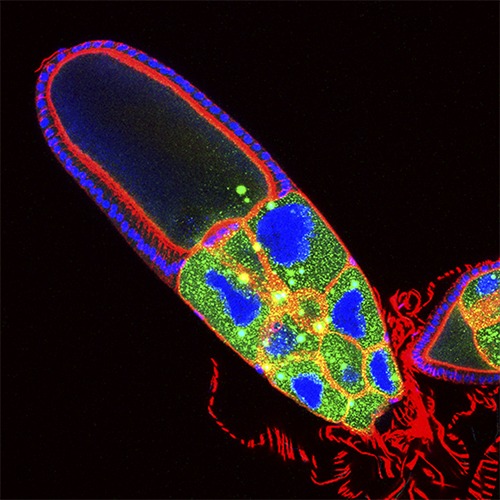
Maintenance
Centrioles and cilia are very stable structures, being resistant to microtubule depolymerising drugs. However, they disassemble in a regulated manner in certain circumstances: upon differentiation in certain cells, upon cell cycle re-entry in others. We uncovered that the matrix that surround the centrioles (PCM) protects them from being eliminated in cultured cells and in eggs, during oogenesis (Marques, Bento et al, Science, 2016). In fact, centriole elimination during oogenesis is critical for correct centriole number to be established upon fertilization.
We are investigating how the PCM provides stability to the centrioles, whether this is differently regulated in different cell types and the possible consequences of its mysregulation for the organism. Moreover, we are also investigating how basal body and cilia maintenance is regulated in Drosophila sensory neurons.
-
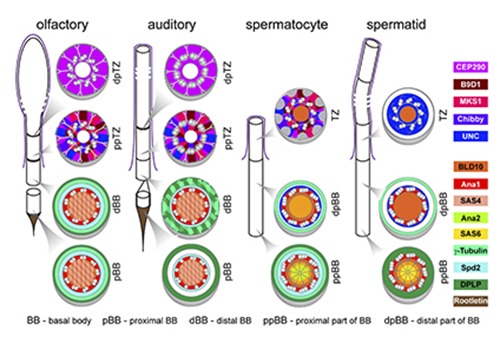
Evolution and diversity
Centrioles and cilia are very conserved structures. Our laboratory has been studying cilia and centrosomal structures and their components in different organisms throughout the eukaryotic tree of life (inter-species diversity) to understand how these structures evolved.
We are also very interested in how abnormalities in those structures evolve during disease, in particular in cancer.
Moreover, we are very interested in how diversity of cellular structures originates within an organism (Intra-species diversity), in particular how different centrosome and cilia types originate and can perform highly specialised and different functions.
-
Disease
Centrosome and cilia alterations, in particular the presence of multiple centrioles, can lead to abnormalities in chromosome segregation, asymmetric cell divisions, invasiveness and ability to induce cancer in mice.
Multiple questions remain, in particular how those changes originate, how they contribute to cancer and how can they be taken advantage off for translational purposes. We use different experimental systems to tackle those questions.
We have dissected when centrosome abnormalities arise in tumor progression (Lopes et al, JCB, 2018), and uncovered that the over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation (Marteil et al, Nature Comm, 2018). Using cancer cell lines, including the NCI-60 panel of human cancer cell lines derived from 9 distinct tissues, we continue to address the highlighted questions combined with bioinformatics and modelling of centriole number along tumor progression.
- 1

